What is PDX Model and the Application of PDX Model
Tumorgenesis is a dynamic process caused by many intertwined factors. During cancer progression, genetic and epigenetic aberrations lead to dramatically different ...

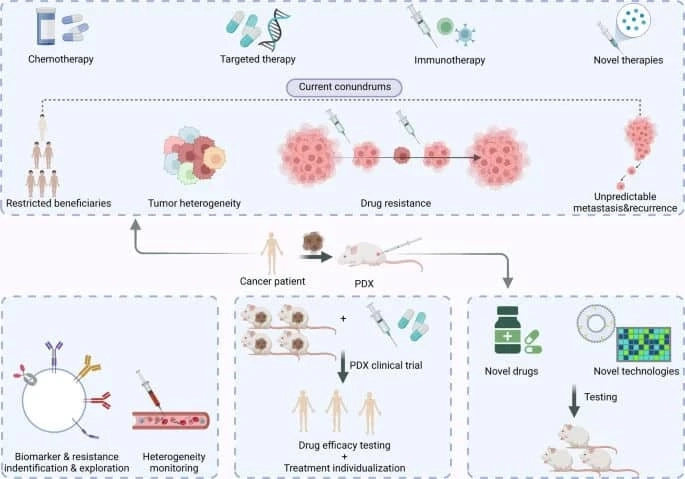
Tumorgenesis is a dynamic process caused by many intertwined factors. During cancer progression, genetic and epigenetic aberrations lead to dramatically different ...

