The healthcare industry is rapidly evolving, driven by digital innovations that enhance patient care, efficiency, and safety. Among these innovations, Software as a Medical Device (SaMD) has emerged as a critical component of modern healthcare technology. SaMD refers to software that performs medical functions independently of physical medical devices, providing diagnostic, monitoring, or therapeutic capabilities. Understanding what is Software as a Medical Device and its applications is essential for healthcare providers, developers, and regulators aiming to navigate the digital health landscape.
Defining Software as a Medical Device
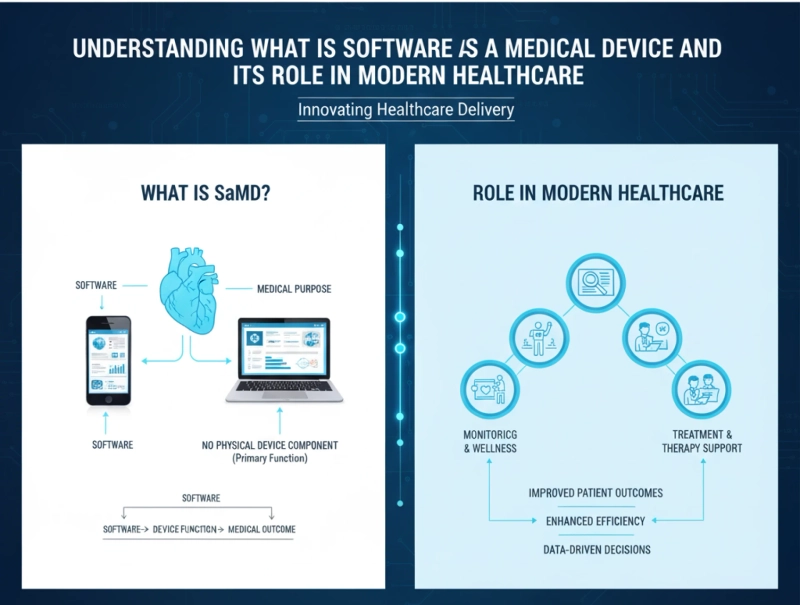
Software as a Medical Device (SaMD) is any software intended to perform one or more medical purposes without being part of a physical medical device. Unlike embedded software, which is integrated within medical equipment, SaMD operates independently to analyze patient data, support clinical decisions, or provide treatment guidance.
According to the International Medical Device Regulators Forum (IMDRF), SaMD must meet specific criteria to be classified as a medical device, including providing a medical purpose, impacting patient care, and requiring regulatory oversight for safety and effectiveness.
Why SaMD Is Important in Healthcare
The rise of digital health and telemedicine has made SaMD a vital tool in modern healthcare systems. It offers numerous advantages that enhance both clinical and operational efficiency:
- Improved Diagnostic Accuracy: AI-powered SaMD can analyze medical imaging, lab results, or patient data to detect diseases early and accurately.
- Remote Monitoring and Care: SaMD platforms can track chronic conditions such as diabetes, cardiovascular disorders, and respiratory illnesses from home.
- Clinical Decision Support: These solutions provide actionable insights, alerts, and recommendations, helping physicians make data-driven decisions.
- Cost Reduction: By automating processes and reducing the need for physical interventions, SaMD helps healthcare organizations lower operational costs.
- Scalable Solutions: Cloud-based SaMD systems can be deployed across hospitals, clinics, and telehealth platforms without extensive hardware investment.
By enabling these capabilities, SaMD is transforming patient care, reducing hospital readmissions, and improving overall health outcomes.
Core Features of Software as a Medical Device
SaMD solutions are distinguished by several key features that make them effective and reliable:
- Medical Purpose: SaMD must have a specific health-related function, such as diagnosis, prevention, monitoring, or treatment.
- Independent Functionality: It performs medical tasks without being part of a physical device.
- Data Integration: SaMD analyzes patient data from multiple sources, including wearable devices, EHRs, and lab results.
- Regulatory Compliance: These solutions must comply with standards such as ISO 13485, IEC 62304, and regional regulations like FDA or European MDR.
- User-Centric Design: SaMD is designed to provide clear, actionable outputs for healthcare providers or patients.
These features ensure that SaMD maintains high accuracy, reliability, and safety in patient care.
Applications of Software as a Medical Device
SaMD has a wide range of applications across different areas of healthcare:
- Diagnostic Imaging: AI-driven software analyzes X-rays, MRIs, and CT scans to detect early signs of disease.
- Chronic Disease Management: Mobile applications monitor conditions such as diabetes, hypertension, or asthma, providing alerts and care recommendations.
- Telehealth Platforms: SaMD enables remote patient consultations, symptom assessment, and virtual monitoring.
- Predictive Analytics: Algorithms forecast disease progression, readmission risks, and potential complications, enabling proactive interventions.
- Mental Health Support: Software delivers cognitive behavioral therapy (CBT) exercises and monitors patient mental wellness.
These applications demonstrate the versatility and potential of SaMD in enhancing healthcare outcomes while reducing the burden on healthcare systems.
Technologies Behind SaMD
Several advanced technologies power SaMD solutions, enabling their functionality and effectiveness:
- Artificial Intelligence (AI) and Machine Learning (ML): AI models analyze large datasets to identify patterns, predict outcomes, and provide decision support.
- Cloud Computing: Cloud infrastructure allows secure data storage, remote accessibility, and scalability.
- Internet of Medical Things (IoMT): IoMT devices feed real-time patient data into SaMD platforms for continuous monitoring.
- Cybersecurity Measures: Encryption, secure authentication, and compliance with HIPAA and GDPR regulations ensure patient data privacy.
- Natural Language Processing (NLP): NLP processes unstructured clinical data from notes, records, and reports to extract meaningful insights.
These technologies enable SaMD to operate efficiently, safely, and in compliance with regulatory standards.
Regulatory Considerations for SaMD
Since SaMD directly impacts patient health, regulatory oversight is critical. Regulatory bodies, including the FDA, European Medicines Agency (EMA), and IMDRF, provide guidelines to ensure SaMD safety and efficacy. Key considerations include:
- Classification: SaMD is categorized based on risk levels, ranging from low-risk wellness software to high-risk diagnostic or therapeutic applications.
- Clinical Validation: Developers must provide clinical evidence demonstrating software accuracy and reliability.
- Post-Market Surveillance: Continuous monitoring ensures ongoing performance and patient safety.
- Documentation: Comprehensive design, testing, and risk management documentation is required for approval.
Compliance with these regulations not only ensures patient safety but also builds trust among healthcare providers and users.
Challenges in Developing SaMD
Despite its potential, developing SaMD comes with challenges:
- Regulatory Complexity: Navigating international standards can be time-consuming and resource-intensive.
- Data Privacy and Security: Handling sensitive patient information securely is paramount.
- Interoperability: Ensuring SaMD works with existing healthcare systems and devices can be complex.
- Algorithm Bias: AI-based SaMD may produce inaccurate results if trained on biased or incomplete datasets.
- User Adoption: Healthcare professionals require training to effectively integrate SaMD into workflows.
Addressing these challenges requires a combination of technical expertise, regulatory knowledge, and user-focused design.
The Future of Software as a Medical Device
The future of SaMD is promising, driven by advancements in AI, IoT, and cloud technologies. Emerging trends include:
- AI-Powered Diagnostics: More accurate and faster disease detection using machine learning models.
- Remote Patient Care: Continuous monitoring of chronic diseases and post-surgical recovery from home.
- Personalized Medicine: Tailoring treatment plans based on patient-specific data and predictive analytics.
- Integration with Wearables: Continuous real-time data collection from smart devices feeding SaMD platforms.
- Explainable AI: Transparent algorithms that provide understandable recommendations to healthcare providers.
These innovations will make healthcare delivery more efficient, precise, and patient-centered.
Conclusion
Understanding what is Software as a Medical Device is essential for anyone involved in modern healthcare. SaMD empowers clinicians with actionable insights, improves patient outcomes, and supports the growing need for digital health solutions. By leveraging advanced technologies like AI, cloud computing, and IoMT, SaMD is transforming healthcare delivery into a more intelligent, connected, and efficient system.
As the industry continues to embrace digital transformation, SaMD will play an increasingly pivotal role in providing safer, smarter, and more personalized care for patients worldwide.