The eye is a highly compartmentalized organ, and its anatomical structure has many self-protective barriers, which also constitute a barrier for drug delivery. The eye is divided into an anterior segment and a posterior segment. The anterior segment includes the cornea, conjunctiva, iris, aqueous humor, and ciliary body, and the posterior segment includes the vitreous, retina, choroid, and sclera. The physiological barriers of the eye include the cornea and conjunctiva barrier, the blood aqueous humor barrier, and the blood-retinal barrier, among which the cornea and retina are barriers that are difficult for drugs to penetrate. [1]
Most ophthalmic drugs sold in the market are in the form of eye drops, eye ointment and ophthalmic gel for external use, which have extremely low bioavailability and basically do not enter the posterior segment of the eye. [2] In addition to traditional ophthalmic solutions, emulsions, suspensions, gels, and ointments, increasing interest has shifted to the development of new, advanced ophthalmic vehicles, including nanomicelles, nanoemulsions, lipids, and bodies, nanosuspensions, in situ gels, etc. Lipid nano-preparations include nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers, and liposomes, and their application in ophthalmology has developed rapidly in recent years.
Medicilon’s ophthalmic drug preparation services cover four types: eye drops, injections, gels, and eye ointments. It has completed safety research on the preparations of various eye drops and vitreous injection new drugs and helped to obtain clinical approval.
Injectable ophthalmic drugs encounter more obstacles in the R&D stage than other dosage forms due to the difficulty of administration. Realize unique fine drug delivery, including: subretinal injection, suprachoroidal injection, vitreous injection, etc., Medicilon uses years of rich practical experience and advanced equipment to solve various complex problems of customers about ophthalmology research, focus on details and control Quality, to provide customers with stable and high-quality research services.
Advantages of Nanoemulsion
Nanoemulsions are transparent, kinetically stable preparations, and the internal phase droplets are generally 20-200nm (the maximum limit can be up to 500nm). Ophthalmic o/w nanoemulsions consist of a dispersed phase (oil), a continuous phase (water), and carefully selected surfactants and cosurfactants, which can lower the surface tension of the two immiscible phases of the nanoemulsion.
In addition, ophthalmic nanoemulsions also need to add bacteriostats, tonicity regulators, buffers, viscosity modifiers, antioxidants, and API solubilizers. These ingredients are intended to increase the physical stability of the formulation and improve physicochemical properties (e.g., reduce droplet size or increase colloidal stability).
Nanoemulsions can improve ocular bioavailability due to their ability to prolong pre-corneal retention time and improve penetration. Surfactants and cosurfactants present in nanoemulsions can act as penetration enhancers for drug corneal delivery. They remove the mucus layer, disrupt the tight junction complexes, and allow the penetration of the API into the deep regions of the eye.
In addition, ocular absorption of drugs in nanoemulsions may improve transport to corneal epithelial cells by endocytosis due to the nanoscale size of the particles.
Because nanoemulsions increase the ocular absorption of drugs, compared with traditional eye drops, the number of dosing can be reduced, which ultimately simplifies the dosing schedule and improves patient compliance.
Therefore, nanoemulsions can achieve the efficacy of ophthalmic formulations using simplified, less frequent administration than conventional ophthalmic formulations.
But nanoemulsions present many challenges due to their multicomponent nature, complex chemistry, and optimization required for robust, stable formulations. Laboratory-scale methods are also very different from industrial-scale methods, difficult to scale up, etc.
Physicochemical evaluation of ophthalmic nanoemulsion
Several characterization methods have been developed to assess the properties and quality of ophthalmic nanoemulsions. These methods enable the study of a formulation\'s physicochemical properties (e.g., appearance and transmittance, particle size distribution and polydispersity index, zeta potential, refractive index, pH, osmotic pressure, surface tension, viscosity), its pharmaceutical properties (e.g., API Assay and in vitro release of nanoemulsions, evaluation of sterility and stability after storage, studies of API/formulation component interactions), and biological effects (e.g., mucoadhesive strength, cytotoxicity, irritation, transcorneal penetration, Antifungal activity, histological examination of the eye after administration and biodistribution of the drug in ocular tissues, pharmacokinetics). Because nanoemulsions are complex multicomponent systems, it is necessary to combine complementary characterization techniques in order to understand formulation performance and clinical outcomes. We will introduce the most commonly used nanoemulsion characterization methods, starting from the determination of the physicochemical properties of the formulation to the evaluation of its in vivo and ex vivo drug performance and biological effects.
1. Visual inspection and light transmittance test
Visual evaluation of nanoemulsions was performed under diffuse daylight against white and black backgrounds to determine the clarity of the formulations to be tested. Depending on the size of the dispersed phase droplets relative to the wavelength of light (380nm < λ < 780nm), the formulation can be a transparent formulation with droplet d < 50nm, or a turbid formulation with droplet size in the range 50nm < d < 200nm. The degree of light scattering in nanoemulsions is a function of the amount of dispersed phase, the droplet size and the refractive index of the dispersed particles.
Two types of measurement equipment were used to analyze the optical properties of nanoemulsions: UV-Vis spectrophotometer and colorimeter. The ultraviolet-visible photometer measures the transmission or reflection of light in the visible wavelength range of 380-780 nm (a single-point measurement is performed at 520 nm, and the light transmittance T of the preparation to be tested is determined, expressed as a percentage (%)). Higher light transmittance (closer to 100%) indicates that the developed system is transparent and suitable for application to the eye. Formulations with low transmittance have limited use in ophthalmic administration because application to the eye may interfere with vision. During clarity analysis, the formulation to be tested is diluted with the selected solvent and analyzed at the appropriate wavelength (λmax) against a reference solvent.
2. Particle size distribution of nanoemulsion
The particle size of the dispersed particles is in the range of 1−500 nm, which significantly increases the stability of the emulsion after preparation and the contact area between the API and the eyeball surface. The absorption of the drug after administration can thus be improved. In addition, the reduction in the droplet size of the dispersed phase in the nanoemulsion increases the penetration of the drug into the deep layers of the eye, including body fluids. The droplet size and polydispersity of nanoemulsions can be determined at room temperature using dynamic light scattering (DLS), photon correlation spectroscopy (PCS), or microscopy methods.
3. Dynamic Light Scattering (DLS), Synchronous Photon Correlation Spectroscopy (PCS) or Quasi-Elastic Light Scattering (QELS)
DLS is the most commonly used technique for determining the average particle size and polydispersity index of the dispersed phase of nanoemulsion systems. In the DLS method, a laser beam is scattered on the particles present in the solution, and on the basis of analyzing the change in light intensity, the average particle size and distribution of the particles are determined. The method used in the measurements monitors the variability of laser light scattering as a function of time due to the Brownian motion of the particles, as small particles move through the solution at higher velocities. Size determination of nanoemulsions is performed on concentrated (undiluted) samples or samples diluted with the external phase (i.e. water). When analyzing formulations containing excipients that increase the viscosity of the system, it is generally necessary to dilute, otherwise the assessment of the droplet size of the dispersed phase will be hindered. It has been reported to dilute 40 to 100 or even 500 times with deionized water. Any specific viscosity value needed to obtain a true value for droplet diameter was not found.
4. Microscopy
To determine the particle size, particle morphology and microstructure of nanoemulsion systems, optical or polarizing microscopes with high-resolution adapters can be used. Lim et al. used an optical microscope with a high-resolution adapter for hyperspectral photography to determine the morphology of nanoemulsion dispersions applied to the eyeball, allowing quantitative determination of the particle size distribution and aggregation of nanoparticles in the formulation to be tested. Hyperspectral imaging is a technique derived from standard digital photography in which images are recorded in channels corresponding to three wavelength ranges, blue, green, and red. Hyperspectral images may consist of hundreds of images captured at tightly defined wavelengths, providing more information about objects than conventional digital photography. The potentially high spectral resolution of this technique (2 nm and above) allows the presentation of recorded data as two-dimensional maps of reflectance spectra, which opens up possibilities for quantitative and qualitative analysis of the particle size distribution of nanoemulsions.
Morsi et al. used polarized light microscopy to determine the presence of liquid crystal lamellar phases or the possibility of API crystallization to assess the stability of nanoemulsions. Although optical microscopy can provide some insight into the structural features of nanoemulsions, high-resolution electron microscopy techniques including transmission electron microscopy (TEM), cryo-TEM, scanning electron microscopy (SEM) or atomic force microscopy (AFM) can also be used to achieve more detailed characterization, enabling imaging of dispersed particles at nanometer resolution.
Dukovski, et al. Functional Ibuprofen-Loaded Cationic Nanoemulsion:Development and Optimization for Dry Eye Disease Treatment. Int.J. Pharm. 2020, 576, 118979.
Dukovski et al. used atomic force microscopy and fluorescence microscopy to determine the morphology of the dispersed phase in ibuprofen-loaded cationic ophthalmic nanoemulsions. Anjana et al. used TEM to analyze the particle size and morphology of curcumin-based ophthalmic nanoemulsions. A dry drop of a 1:100 water-diluted nanoemulsion was deposited on the membrane grid and imaged directly. Shah et al. performed TEM imaging of droplets of moxifloxacin nanoemulsions, which were first stained with phosphotungstic acid solution (2% w/v), then fixed on copper grids, and incubated at room temperature (25±2°C) dried and then subjected to TEM imaging. While classical electron microscopy techniques (TEM or SEM) require staining and/or drying of the nanoemulsion, which can affect nanodroplet size and shape, cryo-EM offers direct imaging of the obtained colloidal system without major structural changes unique opportunity. Although this approach has not been widely used for the characterization of ophthalmic nanoemulsions, it will certainly be adopted in the near future, providing new insights into the structure of colloidal nanoscale drug delivery systems.
Brito, L. A. et al. A Cationic Nanoemulsion for the Delivery of Next-Generation RNA Vaccines. Mol. Ther. 2014, 22 (12), 2118−2129.
5. Zeta potential
Zeta potential is defined as the potential difference (ΔV) between the dispersion medium (water in this case) and the immobilized fluid layer attached to the dispersed oil nanodroplets. The value of this parameter affects the colloidal stability of the designed nanoemulsion. Zeta potential measurements are performed using undiluted or diluted preparations (for example, with KCl solution or water with a specific conductivity). Dilution of the nanoemulsion with water simulated the effect of tear fluid after instillation in the eye, although it should be emphasized that the positive charge on the droplet should not change after dilution. Zeta potentials of neutral nanoparticles in dispersed systems range from −10 to +10 mV. Zeta potentials greater than +30 mV indicate the presence of strongly cationic nanoparticles, while values below −30 mV characterize strongly anionic nanoparticles. Nanoemulsion formulations showing zeta potential values above +30 mV or below −30 mV were considered stable. It should be emphasized that the charge of the droplets in the nanoemulsion may also affect its absorption after application to the eyeball. This is due to the negatively charged surface of the cornea binding positively charged particles. This may prolong the contact time of the drug with the cornea and improve its bioavailability.
6. pH measurement
pH measurements were performed at 25°C using the potentiometric method, and standard buffer solutions at pH 4.0, 7.0, and 10.0 were used for electrode calibration. The pH of the nanoemulsion should correspond to the physiological value of tear fluid, with a pH range of 7.0-7.4 to ensure comfortable application of the formulation. Fluids that deviate significantly from acceptable values may irritate the eye, lead to hypersecretion of tears and consequently rapid flushing of the API in the conjunctival sac. Considering the buffering capacity of tears, the pH range may be 3.5-8.5 (European Pharmacopoeia 10.0, 1163). Ammar et al. showed no irritation, good tolerance, and intact rabbit corneal structure after administering doxazole hydrochloride nanoemulsions in the pH range of 4.34−5.42 to rabbit eyes.
7. Refractive index
Refractive index (RI) is an optical property that can be used to describe the isotropy of nanoemulsion formulations and to identify interactions between chemicals and excipients. The inhomogeneous distribution of surfactants at the oil/water interface may lead to their local ordering and the formation of separated phases (i.e., liquid crystals). Liquid crystals form locally ordered structures and generally have a much higher viscosity than bulk nanoemulsions, resulting in anisotropic optical properties of the formulation. When the sample is placed in front of a light source between two crossed polarizers, the sample is observed to be shiny. In contrast, isotropic nanoemulsions are dark under these conditions. In the case of ophthalmic formulations, the measured RI indicates whether the developed formulation affects visual field quality and discomfort after administration. The refractive index of tears is 1.340−1.360, and eye drops with a maximum RF index of 1.476 can be used.
8. Osmotic pressure
Osmolarity is a complex property that depends on the number of molecules dissolved in a solution. The physiological osmolarity of the tear film ranges from 231-446 mOsm/kg during the day. Preparations with an osmolarity below 100 mOsm/kg or above 640 mOsm/kg may cause eye irritation. However, Haße and Keipert obtained an osmolarity range of 1200−2400 mOsm/kg in the rabbit Draize test, which still did not show irritation. Osmolality, measured using an osmometer, is based on the freezing point depression of the solution being tested compared to the pure solvent.
9. Surface tension
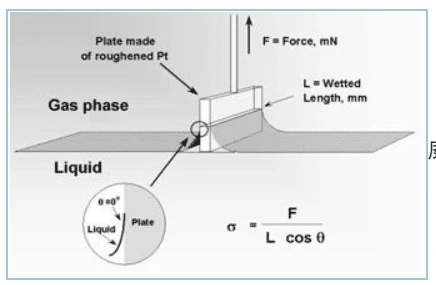
Surface tension is measured using a tensiometer. The method is to measure the force required to lift a submerged Wilhelmy plate or Du Nouy ring from the nanoemulsion surface at a constant temperature. Physiological values of tear surface tension are in the range of 40−50 mN/m. Due to the presence of surfactants, nanoemulsions exhibit low surface tension, which allows the oil phase to be uniformly dispersed in water-based media. In addition, the low surface tension of the formulation may increase corneal wettability and thus drug absorption. Formulations with significantly lower surface tension (i.e. <35 mN/m) compared to tear fluid may cause eye irritation, pain and patient discomfort after application. Conversely, formulations with high surface tension reduce tear film stability.
10. Viscosity measurement
The viscosity of the nanoemulsions is measured at 25°C using a viscometer or rheometer (cone, plate or capillary) at various preset shear rates. Adding appropriate auxiliary substances to increase the viscosity of eye drops is a common method to prolong the contact time of the drug with the eyeball, thereby improving the bioavailability of the drug. The measured viscosity of physiological tears is approximately 1.5 mPa s, and the desired viscosity of nanoemulsions applied to the eyeball should not exceed a maximum of 20 mPa s, as high-viscosity emulsions may occlude the lacrimal duct.
11. Determination of active substance content
The determination of the drug content in the nanoemulsion was carried out by organic phase extraction. The indicated amount of nanoemulsion (e.g., 1 mL) is mixed with the organic phase (e.g., methanol), sonicated, and centrifuged at high speed. The obtained supernatant was analyzed for drug content. To test the efficiency of drug incorporation into nanoemulsions, various extraction methods have been proposed, including ultrafiltration, ultracentrifugation, gel filtration, and microdialysis. Drug incorporation efficiency is related to its properties (ie, lipophilicity, molecular weight, and structure). To determine the shelf life of the product, evaluate the API content in the nanoemulsion (previously performed a stability test) using an appropriate analytical technique (i.e. HPLC or spectrophotometry).
12. Sterile
Sterility is one of the basic requirements for ophthalmic preparations. Sterility testing determines the presence of bacteria and fungi in a given formulation. Chinese Pharmacopoeia 2020 Edition 1101 Sterility Test. A method for checking the sterility of pharmaceuticals, biological products, medical devices, raw materials, excipients and other varieties that require sterility in the Pharmacopoeia.
13. Stability study
Nanoemulsions are thermodynamically unstable and may undergo flocculation, coalescence, Ostwald ripening and phase separation during storage. Therefore, the final formulation of nanoemulsions must remain physically and chemically stable under ambient conditions during production, storage, transportation, and application. Due to changes in pH, ionic strength, temperature, and mechanical force, the properties of nanoemulsions change, which may lead to their destabilization, particle size distribution, and morphology changes, which in turn affect the release of dispersed phase substances.
Stability assessment methods can be based on the observation of emulsion systems over a specified period of time (emulsion aging method) and also allow for a rapid assessment of the durability of formulations (accelerated stability test method). Long-term stability studies of nanoemulsions enable real-time stability assessments.
During the long-term stability study, the formulations were stored at different specific temperatures for 3-6 months, and the properties of the nanoemulsions, namely viscosity, pH, refractive index, average droplet size and bulk drug substance were tested at different sampling time points. content. Stable formulations are characterized by no phase separation, a clear appearance and only slight changes in physicochemical parameters.
Accelerated stability studies, i.e. centrifugation and thermal testing, are also used to assess the stability of nanoemulsions. These methods are designed to accelerate the development of robust formulations during pre-formulation studies under strict time frames.
Medicilon Ophthalmic Preparation Development Platform
The Medicilon Preparation Department can undertake the development of dosage forms including ophthalmic liquid preparations and ophthalmic semi-solid preparations. Medicilon has solutions, suspensions, emulsions, gels, ointments, creams and other technologies. platform. The completed project categories include eye drops 1, 2, and 4, all of which have been successfully declared and are currently undergoing clinical trials. At the same time, Medicilon can undertake preclinical research in ophthalmology. The ophthalmology platform has a special intraocular drug delivery technology and is equipped with an advanced ophthalmic surgery microscope. Animal species such as dogs, miniature pigs and non-human primates achieve unique fine-grained dosing.
Medicilon Heidelberg laser ophthalmology diagnostic instrument SPECTRALIS®HRA + OCT
References:
[1]. Jin Yiguang et al. Application of nanotechnology in drug delivery.
[2] Zhang Shanshan, Zhu Jing, Zhao Yongyue, etc. Ophthalmic drug delivery technology and its research and development overview [J]. Chinese Journal of Clinical Pharmacology, 2015, 31(07): 580-583. DOI: 10.13699/j.cnki. 1001-6821.2015.07.028.