In 1883, E. Fischer and F. Jourdan treated pyruvate with 1-methylphenylhydrazone with HCl alcohol solution to obtain 1-methylindole-2-carboxylic acid. After this discovery, the Fischer indole synthesis reaction became a very significant method for the synthesis of substituted indoles. The Fischer indole synthesis reaction is a simple and rapid synthesis method for arylhydrazones obtained from the reaction of aldehydes or ketones with arylhydrazones.
The drug discovery of Medicilon mainly include chemistry (synthetic chemistry, medicinal chemistry) and biology. Among them, our chemistry team can undertake multi-dimensional business cooperation such as custom synthesis of compunds, compound library construction, SAR compound synthesis and screening, compound structure and biological activity optimization.
This reaction is followed by cyclization to generate polysubstituted indoles. The phenylhydrazone generated in the reaction is heated and rearranged under acid catalysis to eliminate a molecule of ammonia to obtain 2-substituted or 3-substituted indole derivatives. In practical operation, aldehyde or ketone and equivalent phenylhydrazine can be heated to reflux in acid to obtain phenylhydrazone, which can be rearranged immediately under acid catalysis to eliminate ammonia to obtain indole compounds. The commonly used catalysts are zinc chloride, boron trifluoride, polyphosphoric acid, AcOH, HCl, trifluoroacetic acid, etc. Under the catalysis of Lewis acid, this reaction can usually be carried out under mild conditions, while protic acid usually needs to be heated to higher temperature.
In recent years, with the development of coupling reactions, people have developed a more convenient coupling reaction method. Fisher\'s indole synthesis method has been widely used in the field of alkaloid and pharmaceutical synthesis, even an indispensable method. A synthetic method for aryl hydrazones via the Buchwald-Hartwig coupling of aryl halides and hydrazones was also developed (modified by Buchwald).
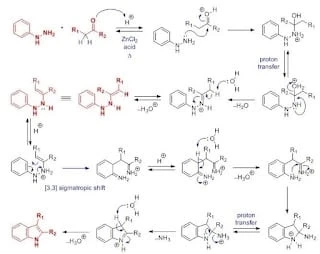
Reaction mechanism
Aldehydes, ketones and aryl hydrazones react, and the generated aryl hydrazones are heated under an acidic catalyst, and undergo a single-electron transfer cyclization to generate substituent indoles.
Response instance
Synthesis of Peduncularine. 【Roberson, C. W.; Woerpel, K. A, J. Am. Chem. Soc. 2002, 124, 11342.】
References
1. Name Reactions (A Collection of Detailed Reaction Mechanisms), Jie Jack Li, Fischer indole synthesis, pages 253-254.
2. Organic Chemistry Portal: http://www.organic-chemistry.org/namedreactions/fischer-indole-synthesis.shtm
3. Chemical station: http://www.chem-station.com/cn/reactions/2014/05/%E8%B4%B9%E8%88%8D%E5%B0%94%E5%90%B2% E5%93%9A%E5%90%88%E6%88%90-fischer-indole-synthesis.html