Antibody-Drug-Antibody (ADC)
Each ADC R&D project is its own challenge due to the varieties in the assembly of ADC molecules. With this concept in mind, Medicilon promises careful planning, meticulous execution and accurate results through years of practical experience and effective communication with our clients.
Synthesis of ADC Payloads
Medicilon offers clients with a pool of payload drugs of various mechanisms. We also offer custom synthesis of payload drugs as clients demands.
Pharmacology Evaluation of ADC
One important pharmacological parameter of an ADC is the in vivo efficacy that directly reflects its potency and influences clinical trial designs. Our animal models are all established and maintained under the regulation of AAALAC. We conduct our tests with GLP-like standards.
We have established more than 200 types of oncological models for ADC efficacy assessment.
- Tumor models for multiple tumor diseases
- Diverse selections of model types
- Xenograft models
- Syngeneic models
- Orthotopic xenograft models
- Transgenic models,
- hPBMC/CD34+ HSC humanized models
- PDXC models
- Various laboratory animal
- Rodents
Mouse/Rat, Rabbit - Non- Rodents
Beagle Dog, Mini Pig, Non-human Primate
- Rodents
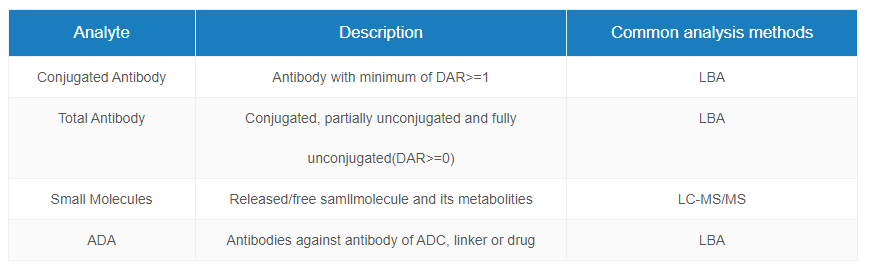
ADC Pharmacokinetics Study
ADC raises the difficulties of PK study for each component ADC molecules owning unique PK characteristics. Medcilon provides high quality quantification assays for key parameters in ADC PK study, presenting accurate results.

PK and TK

Developing stable and reliable methods for results with high correlation.

Benchmarking with global lab standard for results with high consistency.
ADC immunogenicity
Immunogenicity is a key parameter when evaluating biologic therapeutics. It could increase the risk for adverse effect and reducing ADC efficacy. Medicilon fully understands the compexity of ADA evaluation and offers our clients with comprehensive immunogenicity assays.
ADC Safety assessment
Medicilon offers rigorous and specific safety assessment services strictly following S6 & S9 Regulation of ICH and in compliance with the requirement of NMPA, FDA, OECD and TGA.
· Single dose/Repeat dose toxicity (With TK)
· Tissue cross-reactivity
· ADA test
