Aim at chemical synthesis through Ibuprofen
Recently, Pfizer’s new crown-specific drug Paxlovid and the national miracle drug Ibuprofen are the most popular drugs in the medical circle. Today we will talk about the synthesis and mechanism of action of Ibuprofen, an evergreen drug! Recently, Ibuprofen has been hard to find, and it has become a well-known hard currency.
Ibuprofen
Ibuprofen, molecular formula C13H18O2, chemical name 2-(4-Isobutylphenyl) propanoic acid. Ibuprofen is a commonly used non-steroidal anti-inflammatory drug (NSAID). By inhibiting cyclooxygenase and reducing the synthesis of prostaglandins, it produces analgesic and anti-inflammatory effects; it acts as an antipyretic through the hypothalamic thermoregulatory center effect. Ibuprofen is generally well tolerated and does not cause clinically significant acute liver injury.

Ibuprofen chemical struture
Indications for Ibuprofen
Oral administration: used to relieve mild to moderate pain, such as arthralgia, neuralgia, muscle pain, migraine, headache, dysmenorrhea, and toothache, and also reduces fever caused by the common cold or influenza.
Injection administration: for antipyretic and analgesic treatment: treatment of mild to moderate pain, adjunct to opioid analgesics for treating moderate to severe pain; antipyretic therapy of fever.
Mechanism of Action of Ibuprofen
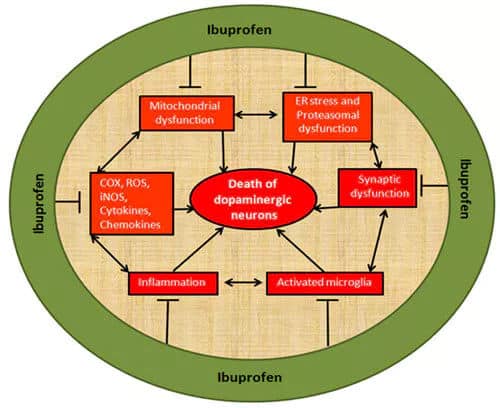
Ibuprofen is a diastereoisomeric chiral drug, including R-ibuprofen and S-ibuprofen. Pharmacokinetic studies indicate that most of the pharmacologically active form of the drug is contributed by its S-enantiomer, which inhibits prostaglandin synthesis. A unidirectional enzymatic conversion of the inactive R-enantiomer to the active S-enantiomer was first reported in the early to mid-1970s. Approximately 40-60% of the R-enantiomer is metabolized in the liver to the S-ibuprofen form. Its anti-inflammatory effect is due to the inhibitory activity of S-ibuprofen on cyclooxygenase (COX), which is responsible for the synthesis of prostaglandins and plays an essential role in neuronal death mediated by exacerbated inflammatory responses. Ibuprofen inhibits COX, reduces nitric oxide (NO) production, and activates peroxisome proliferator-activated receptor-gamma (PPAR-gamma).

Mechanism of action of Ibuprofen [1]
Chemical Synthesis of Ibuprofen
Ibuprofen was first patented by Boots Pure Chemical Company in 1961 and approved as an over-the-counter drug in 1984. Since its inception, Ibuprofen has been sold under several brands, including Advil® and Motrin®. The synthesis of Ibuprofen starts from isobutyl benzene. The synthesis procedure includes Friedel-Crafts acylation, reduction, chloride substitution, and Grignard reaction. The products of each step were then analyzed by IR and 1 H NMR spectroscopy, and the final product was confirmed by analysis of melting points.
In 1992, BHC Corporation developed a new sustainable synthesis method that cut the synthesis steps in half. The first step uses anhydrous hydrogen fluoride as a catalyst and solvent, which is then recycled for reuse. Furthermore, the natural beauty behind the BHC method is the reduction of unwanted waste and impurities. Only one molecule of water is produced as a by-product, making for a genuinely green synthesis.

Chemical Synthesis of Ibuprofen[2]
In 2009, Andrew R. Bogdan et al. from Florida State University synthesized Ibuprofen using a microreactor. Microreactors are safer, more efficient, and more selective chemical transformations in microchannels or narrow-bore tubes. Using isobutyl benzene and propionic acid as starting materials, the total reaction time was about 10 min, and the yield was 68% crude and 51% after recrystallization (purity 99% by GC and NMR analysis).

The continuous synthesis process of Ibuprofen [3]
In 2014, Dr. David R. Snead and Professor Timothy F. Jamison of Massachusetts Institute of Technology (MIT) performed a multi-step continuous reaction using isobutyl benzene and propionyl chloride as raw materials. Ibuprofen was assembled from its basic building blocks in three minutes, with an average yield of more than 90% per step from the starting material to the target compound.

The continuous synthesis process of Ibuprofen [4]
References
[1] Ashish Singh, et al. Neuroinflammatory responses in Parkinson’s disease: relevance of Ibuprofen in therapeutics. Inflammopharmacology. 2021 Feb;29(1):5-14. doi: 10.1007/s10787-020-00764-w.
[2] Mark A. Murphy. Early Industrial Roots of Green Chemistry and the history of the BHC Ibuprofen process invention and its Quality connection. Foundations of Chemistry volume 20, pages121–165 (2018).
[3]Andrew R Bogdan, et al. The continuous-flow synthesis of Ibuprofen. Angew Chem Int Ed Engl. 2009;48(45):8547-50. doi: 10.1002/anie.200903055.
[4] David R Snead , et al. A three-minute synthesis and purification of ibuprofen: pushing the limits of continuous-flow processing. Angew Chem Int Ed Engl. 2015 Jan 12;54(3):983-7. doi:
